Use of the long-acting opioid pain medication Belbuca may increase the risk of tooth decay and other serious dental side effects, according to a warning from the U.S. Food and Drug Administration (FDA).
What You Can Do & How We Can Help
The Schmidt Firm, PLLC is currently evaluating cases where a Belbuca user has taken the medication for a period of time then suddenly experienced dental problems like pain, chipping, cracking, decay and/or erosion. It is helpful if the patient can show they had good dental health prior to initiating treatment with Belbuca.
If you or somebody you know was diagnosed with severe tooth decay after using Belbuca, you should contact our lawyers immediately for a free case consultation. Please use the form below to contact our Defective Drug Litigation Group or call toll free 24 hours a day at (866) 920-0753.
Belbuca Tooth Decay Lawsuits Consolidated in MDL
In February 2024, dozens of tooth decay lawsuits for buprenorphine medications that dissolve in the mouth were centralized into a Multi-District Litigation (MDL) in the Northern District of Ohio — Case Number 1:24-md-03092.
What is Belbuca?
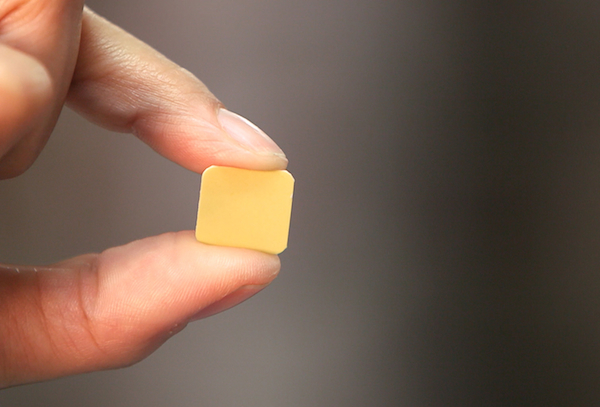
Belbuca contains buprenorphine, an FDA-approved drug used to treat acute pain, chronic pain, and opioid dependence. The medicine works by reducing feelings of pain by interrupting the way nerves signal pain between the brain and the body. Belbuca is manufactured and marketed by BioDelivery Sciences International, and is sold as a buccal film that you place in your mouth and allow to dissolve.
Oral Buprenorphine linked to Severe Tooth Decay, FDA Warns
Belbuca and other orally dissolving medications that contain buprenorphine have been associated with hundreds of cases of severe dental problems, including total tooth loss, the FDA warned in a safety communication [1.] on January 12, 2022.
The oral side effects of buprenorphine include cavities/tooth decay, including rampant caries; dental abscesses/infection; tooth erosion; fillings falling out; and, in some cases, total tooth loss. More than 300 cases of tooth problems have been reported, even in patients with no history of dental problems, according to the FDA.
The agency is requiring a new warning about the risk of dental problems to the prescribing information and the patient medication guide for Belbuca and all other buprenorphine-containing drugs that are dissolved in the mouth.
Belbuca Side Effects
- Tooth decay
- Cracked teeth
- Cavities
- Oral infections
- Tooth loss
- Dental Caries (loss of enamel, dentine, or other tooth substances)
- Root canal
- Tooth extraction
- Crown replacement
What are the Allegations Against Belbuca?
Belbuca lawsuits allege that the drug causes dental decay after extended use. Using Belbuca several times a day over time starts to decay teeth and patients were not adequately warned of this risk, according to the complaints. If Plaintiffs had known Belbuca would cause tooth decay, they would not have used it and would have looked for a safer alternative.
Other Side Effects of Belbuca
In addition to increasing the risk of tooth decay and other serious dental problems, Belbuca may cause the following side effects in some users:
- Headache
- Opioid withdrawal symptoms, such as body aches, abdominal cramps, and rapid heart rate
- Anxiety
- Insomnia (trouble sleeping)
- Sweating
- Depression
- Constipation
- Nausea
- Weakness or fatigue
- Back pain
- Burning mouth syndrome (burning sensation of the mouth or tongue)
- Redness in the mouth
FDA Recommendations
The FDA is recommending that patients who have been prescribed Belbuca or other buprenorphine medications to continue use as prescribed and not stop suddenly without first talking to their doctor, as this could lead to adverse health consequences or even death.
Patients should also be educated on strategies to maintain or improve oral health while taking Belbuca and other similar drugs.
After Belbuca is completely dissolved, the patient should take a large sip of water, swish it gently around the teeth and gums, swallow, and wait at least 1 hour before brushing their teeth. This allows the mouth to gradually return to oral homeostasis and reduces the risk of any mechanical damage that may occur due to brushing.
The FDA also advises that patients tell their provider about any history of tooth problems, including cavities, and schedule a dentist appointment immediately after starting treatment with Belbuca.
Do I Qualify to File a Belbuca Lawsuit?
The Schmidt Firm, PLLC is currently evaluating cases where a Belbuca user has taken the medication for a period of time then suddenly experienced dental problems like pain, chipping, cracking, decay and/or erosion. It is helpful if the patient can show they had good dental health prior to initiating treatment with Belbuca.
If you or somebody you know has experienced tooth decay or any other dental side effects after using Belbuca, you should contact our lawyers immediately for a free case consultation. Please use the form below to contact our Product Liability Group or call toll free 24 hours a day at (866) 920-0753.
Attention Lawyers: We consider a referral from another law firm to be one of the greatest compliments. If your firm is interested in referring us a case or for us to send you a list of previous award judgments and/or average referral fees, please visit the Lawyer Referral section of our website.

 The Schmidt Firm, PLLC has been recognized as one of the nation’s leading plaintiffs' law firms and handles cases in all 50 states. We are very proud of our legal achievements, but equally self-respecting of our firm's reputation for providing personal attention to each and every client we represent.
The Schmidt Firm, PLLC has been recognized as one of the nation’s leading plaintiffs' law firms and handles cases in all 50 states. We are very proud of our legal achievements, but equally self-respecting of our firm's reputation for providing personal attention to each and every client we represent.

