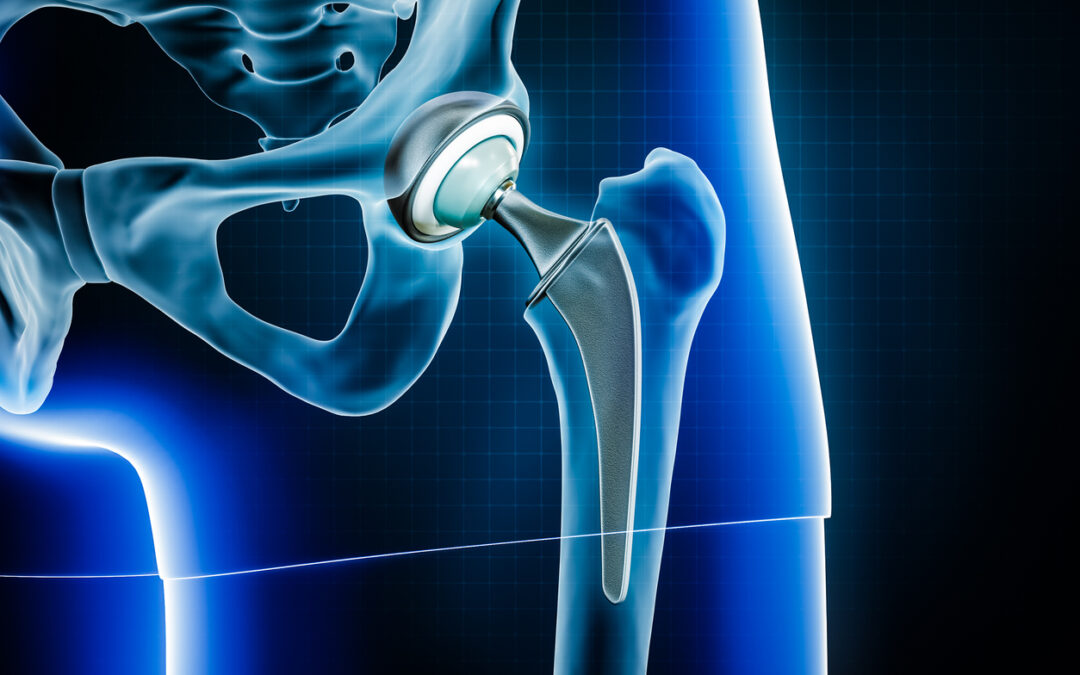
Stryker has announced a hip implant recall for its Rejuvenate Modular and ABGII modular-neck hip stems. The devices have been recalled after being linked to high rates of fretting corrosion at the neck junction, which may cause severe pain, swelling, toxic metal poisoning, and more. Patients who suffer these side effects may need expensive, painful, and debilitating revision surgery.
What You Can Do & How a Stryker Hip Lawsuit Can Help
The Schmidt Firm, PLLC is currently accepting Stryker hip implant induced injury cases in all 50 states. If you or somebody you know has been injured by a defective hip implant, you should contact our lawyers immediately for a free case consultation. Please use the form below to contact our Defective Medical Device Litigation Group or call us toll-free 24 hours a day at (866) 920-0753.
UPDATE: After Settlement, Stryker Hip Lawsuits Continue to be Filed
February 16, 2015 — Stryker Orthopedics is facing a growing number of lawsuits as more people experience failure of the defective Rejuvenate and ABG II hip implants. Click here to read more.
Stryker Settles 4,000 Rejuvenate, ABG II Lawsuits for $1 Billion
November 4, 2014 — Two years after recalling defective Rejuvenate and ABG II hip replacements, Stryker Corp. has announced a $1 billion settlement in 4,000 lawsuits pending in federal court. Click here to read more.
Problems With Stryker Rejuvenate and ABG II
The Stryker Rejuvenate Modular Hip System and the Stryker ABG II Modular-Neck implants have a metal-on-metal component between the stem and neck junction (most other hip implants have a single piece of metal). Unfortunately, over time, this piece can cause fretting and/or corrosion, which releases toxic particles of metal into the patient’s hip. Some patients suffer inflammatory reactions, tissue damage, bone loss, extreme pain, and progressive loosening of the hip implant, which requires revision surgery.
Stryker Hip Implant Recalls in U.S.
- July 4, 2012, Stryker recalled the Rejuvenate Modular and ABGII modular-neck hip stems. The devices were recalled because metal debris could accumulate at the neck junction, potentially causing a local tissue reaction.
Stryker Hip Recall in Canada
April 30, 2012 — Stryker announced a recall of the following devices:
- Rejuvenate Modular Neck, 0 DEG
- Rejuvenate Modular Neck, 8 DEG
- Rejuvenate Modular Neck, 16 DEG
- Rejuvenate SPT Modular Stem, Straight Press-Fit Texture TMFZ
The company cited a failure rate of “less than one percent” due to fretting and/or corrosion around the modular neck junction — the same corrosion issue that prompted the U.S. recall in July.
Stryker Warns Surgeons of Hip Implant Safety Risks
May 2012 — Stryker issued an Urgent Field Safety Notice to surgeons regarding the ABG II Modular Stems and Rejuvenate Modular Necks. The company wanted to warn that fretting and/or corrosion around the modular neck joint could lead to device failure.
Specifically, the company warned about the following hazards:
- Excessive metal debris and/or ion generation from fretting and/or corrosion, which could accumulate in the joint spaces
- Inflammation, metallosis, necrosis (tissue death), and pain caused by contact of the metal ions, tissues, and structures
- Allergic reaction in some patients with heightened metal sensitivity
- Bone loss due to excessive metal debris caused by fretting and/or corrosion.
Stryker Hips Fast-Tracked FDA Approval
Like many new hip implants, Stryker’s Rejuvenate Modular and ABG II modular-neck hip replacements were approved through the FDA’s 510(k) clearance program. This means that if a company can prove that their device is “substantially similar” to a preexisting device that is already approved by the FDA, they do not need to conduct expensive, time-consuming safety studies. Unfortunately, in some cases, “similar” devices have their own unique safety issues.
Symptoms of Hip Implant Failure
- Pain
- Squeakiness or abnormal sounds
- Swelling, inflammation
- Changes in gait or walking ability
- Corrosion
- Dislocation and/or loosening of the hip joint
- Metal poisoning
- Bone loss
- Tissue damage
Do I have a Stryker Hip Implant Lawsuit?
The Schmidt Firm, PLLC is currently accepting Stryker hip implant induced injury cases in all 50 states. If you or somebody you know has been injured by a defective hip implant, you should contact our lawyers immediately for a free case consultation. Please use the form below to contact our Defective Medical Device Litigation Group or call toll free 24 hours a day at (866) 920-0753.
Attention Lawyers: We consider a referral from another law firm to be one of the greatest compliments. If your firm is interested in referring us a case or for us to send you a list of previous award judgments and/or average referral fees, please visit the Lawyer Referral section of our website.

 The Schmidt Firm, PLLC has been recognized as one of the nation’s leading plaintiffs' law firms and handles cases in all 50 states. We are very proud of our legal achievements, but equally self-respecting of our firm's reputation for providing personal attention to each and every client we represent.
The Schmidt Firm, PLLC has been recognized as one of the nation’s leading plaintiffs' law firms and handles cases in all 50 states. We are very proud of our legal achievements, but equally self-respecting of our firm's reputation for providing personal attention to each and every client we represent.

