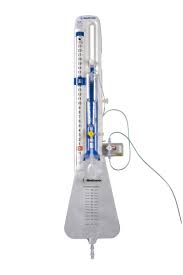
July 2, 2014 — The U.S. Food and Drug Administration (FDA) has issued a Class I recall for the Duet External Drainage and Monitoring System, a device manufactured by Medtronic that drains cerebrospinal fluid and monitors intracranial pressure.
The problem is that the patient line tubing can disconnect from the patient line connectors, and it is probably more common during frequent handling. According to the recall notification:
“The device failure may result in air within the skull (pneumocephalus), infection (such as meningitis, ventriculitis, encephalitis) and over/under drainage of the CSF that may contribute to serious adverse health consequences, including death.”
A spokeswoman for Medtronic told MassDevice that the problem was linked to 35 events from September 2013 through April 2014. No deaths or permanent serious injuries were reported. Medtronic has already addressed the problem and no additional events have been reported.
Recalled products include:
- Interlink Injection Sites, Catalog Number 46913
- SmartSite Injection Sites, Catalog Number 46914
- Interlink Injection Sites, Ventricular, Catheter, Catalog Number 46915
- SmartSite Injection Sites, Ventricular Catheter, Catalog Number 46916
- Interlink Injection Sites, Lumbar Catheter, Catalog Number 46917
Do I have a Duet Neuro Drainage System Lawsuit?
The Schmidt Firm, PLLC is currently accepting Duet Neuro Drainage and Monitoring System induced injury cases in all 50 states. If you or somebody you know has been injured, you should contact our lawyers immediately for a free case consultation. Please use the form below to contact our Defective Medical Device Litigation Group or call toll free 24 hours a day at (866) 920-0753.
Attention Lawyers: We consider a referral from another law firm to be one of the greatest compliments. If your firm is interested in referring us a case or for us to send you a list of previous award judgments and/or average referral fees, please visit the Lawyer Referral section of our website.

 The Schmidt Firm, PLLC has been recognized as one of the nation’s leading plaintiffs' law firms and handles cases in all 50 states. We are very proud of our legal achievements, but equally self-respecting of our firm's reputation for providing personal attention to each and every client we represent.
The Schmidt Firm, PLLC has been recognized as one of the nation’s leading plaintiffs' law firms and handles cases in all 50 states. We are very proud of our legal achievements, but equally self-respecting of our firm's reputation for providing personal attention to each and every client we represent.

