January 17, 2017 — Lawyers have petitioned a panel of federal judges to centralize six lawsuits involving Stryker’s recalled LFIT V40 femoral heads into a Multi-District Litigation (MDL) in Massachusetts.
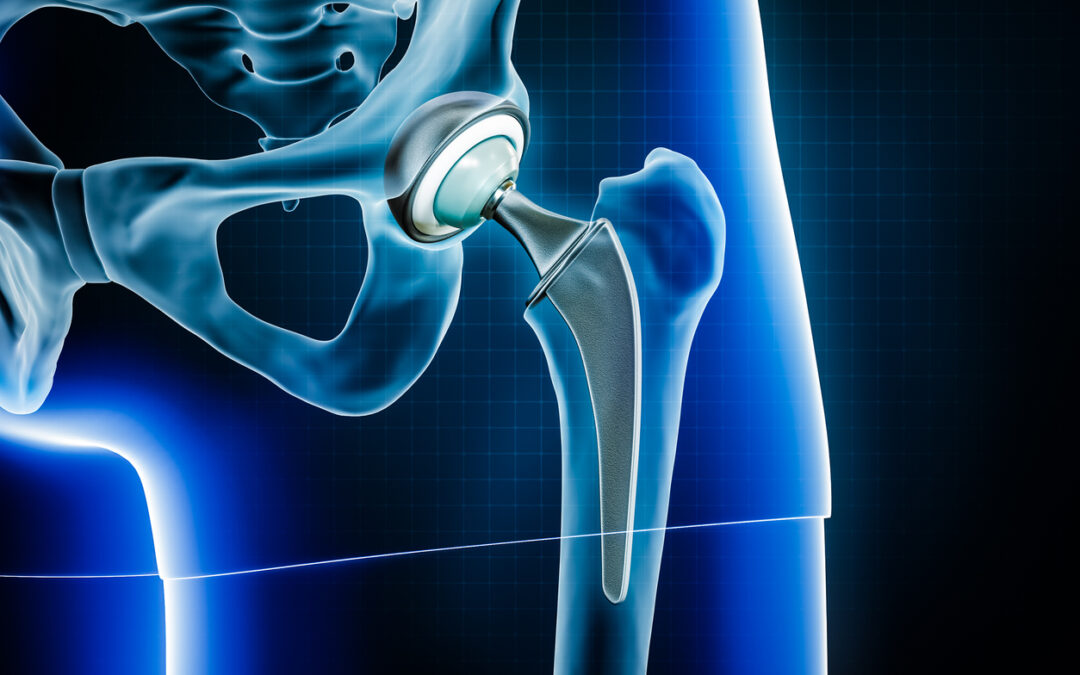
LFIT V40 is a metal femoral head — the “ball” part of a hip — and when it is attached to a metal femoral stem, a metal-on-metal hip replacement is created. This design is problematic because metal particles grind off, accumulate in the hip, and poison the bloodstream. Tens of thousands of lawsuits have been filed by people who were injured.
Last year, Stryker recalled around 45,500 LFIT V40 femoral heads that were sold between 2002 and 2011. In August 2016, Stryker issued an urgent warning about taper lock failures involving LFIT Anatomic CoCr V40 femoral heads manufactured before 2011.
Taper lock failures cause the “ball” part of the hip to dislocate from the hip stem. It can cause loss of mobility, pain, inflammation, tissue death (necrosis), metal poisoning, broken bones, and joint instability.
The most recent lawsuit (PDF) was filed by Patton Witt, a man from Alaska who had his hip replaced in March 2008. His surgeon implanted an Accolade TMZF femoral stem with an LFIT V40 femoral head.
Unfortunately, he developed elevated cobalt levels in his bloodstream, and an MRI demonstrated a large soft tissue mass. Doctors said the problems were likely due to corrosion at the junction between the Accolade TMZF and LFIT V40 and recommended revision surgery.
More problems were discovered during the surgery in January 2015 — metal corrosion, inflammatory changes, adverse local tissue reactions. According to the lawsuit, Witt’s surgeons wrote:
“One could clearly see extensive corrosion present at this site. There appeared to be some deterioration at the trunnion with loss of the passified layer.”
The surgeon found a worn-out metal part where the Accolade TMZF and LFIT V40 rubbed together — a complication called trunnionosis, which is recognized as a growing cause of hip replacement failure and metallosis (metal poisoning).
The lawsuit was filed on January 12, 2017 in the U.S. District Court for Alaska — In RE: Patton Witt, et al. v. Howmedica Osteonics Corp. — Case No. 4:17-cv-00001.
Do I have a Stryker LFIT V40 Hip Replacement Lawsuit?
The Schmidt Firm, PLLC is currently accepting hip replacement induced injury cases in all 50 states. If you or somebody you know has been injured or needed surgery, you should contact our lawyers immediately for a free case consultation. Please use the form below to contact our Defective Medical Device Litigation Group or call toll free 24 hours a day at (866) 920-0753.
Attention Lawyers: We consider a referral from another law firm to be one of the greatest compliments. If your firm is interested in referring us a case or for us to send you a list of previous award judgments and/or average referral fees, please visit the Lawyer Referral section of our website.

 The Schmidt Firm, PLLC has been recognized as one of the nation’s leading plaintiffs' law firms and handles cases in all 50 states. We are very proud of our legal achievements, but equally self-respecting of our firm's reputation for providing personal attention to each and every client we represent.
The Schmidt Firm, PLLC has been recognized as one of the nation’s leading plaintiffs' law firms and handles cases in all 50 states. We are very proud of our legal achievements, but equally self-respecting of our firm's reputation for providing personal attention to each and every client we represent.

