AACE Pharmaceuticals Lubricant PM Eye Ointment was recalled because it may not be sterile, which poses a risk of eye infections, vision loss, and other complications.
What You Can Do & How We Can Help
The Schmidt Firm, PLLC is currently accepting AACE Eye Ointment induced injury cases in all 50 states. If you or somebody you know was diagnosed with a severe eye infection from AACE Eye Ointment, you should contact our lawyers immediately for a free case consultation. Please use the form below to contact our Defective Drug Litigation Group or call toll free 24 hours a day at (866) 920-0753.
What is the Problem?
In February 2024, the India-based pharmaceutical company Brassica Pharma Pvt. Ltd. announced a recall for several types of eye ointments that may not be sterile, which poses a risk of eye infections.
What Happened?
No infections were linked to the recalled eye ointments, but an FDA inspection of the facility in Thane, India discovered a “lack of sterility assurance,” according to the recall.
The recall involves Equate Lubricant Eye Ointment, Equate Stye Lubricant Eye Ointment, CVS Health Lubricant Eye Ointment, and AACE Pharmaceutical Lubricant PM Ointment.
How To Identify Recalled AACE Eye Ointment
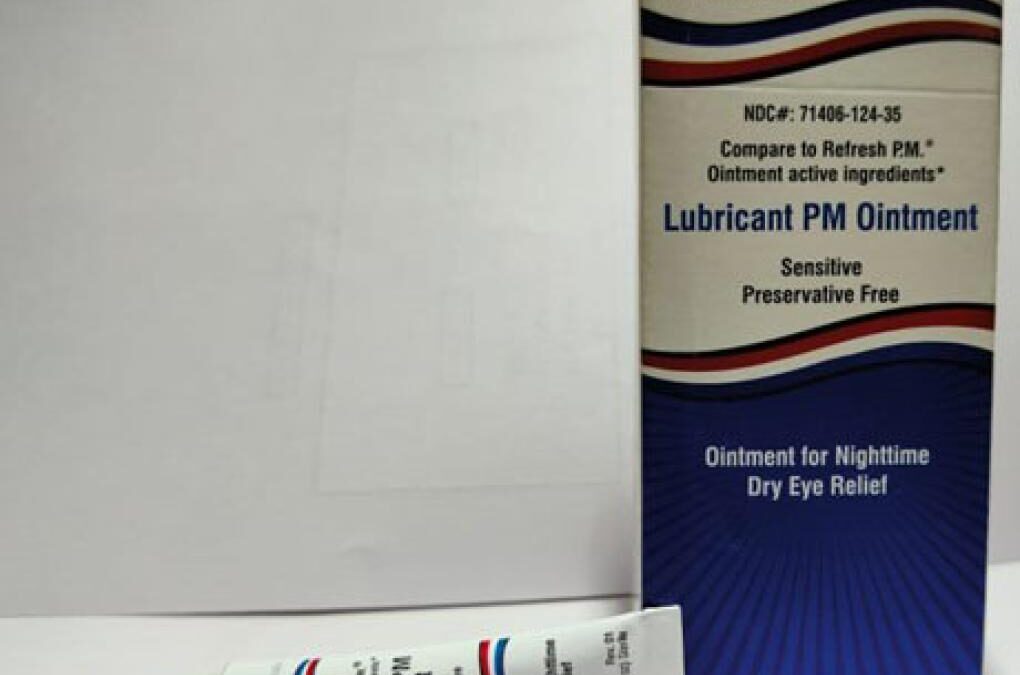
The recall for AACE Pharmaceutical Eye Ointment involves the following product:
- Lubricant PM Ointment: Manufactured by AACE Pharmaceuticals — 3.5 gram tube, packaged in cardboard box with UPC Code 371406124356 with best-by dates between June 2024 and September 2025.
Health Risks Of Using Contaminated Eye Ointments
People who use eye ointments that are contaminated with bacteria or other microbes could develop a severe eye infection. This eye infection could lead to vision loss, blindness, or other complications.
The FDA stepped up enforcement of India-based drug-makers after a deadly outbreak of eye infections was linked to eye drops that were manufactured in India. The eye drops were found to be contaminated with Pseudomonas, an antibiotic-resistant “superbug” bacteria.
The outbreak killed at least 4 people and infected more than 80 other people with Pseudomonas. These infections started in the eyes and became deadly when they spread to the person’s bloodstream.
What is the Risk?
Eye ointments pose a heightened risk because they are applied directly to the eyes, which bypasses some of the body’s natural defenses against infections. Some of the health risks include:
- Eye infections
- Vision loss
- Blindness
- Loss of the eye
- Invasive infection spreads to the body
- Bloodstream infection
- Death
Do I have an AACE Eye Ointment Lawsuit?
The Schmidt Firm, PLLC is currently accepting AACE Eye Ointment induced injury cases in all 50 states. If you or somebody you know was diagnosed with a severe eye infection from AACE Eye Ointment, you should contact our lawyers immediately for a free case consultation. Please use the form below to contact our Defective Drug Litigation Group or call toll free 24 hours a day at (866) 920-0753.
Attention Lawyers: We consider a referral from another law firm to be one of the greatest compliments. If your firm is interested in referring us a case or for us to send you a list of previous award judgments and/or average referral fees, please visit the Lawyer Referral section of our website.

 The Schmidt Firm, PLLC has been recognized as one of the nation’s leading plaintiffs' law firms and handles cases in all 50 states. We are very proud of our legal achievements, but equally self-respecting of our firm's reputation for providing personal attention to each and every client we represent.
The Schmidt Firm, PLLC has been recognized as one of the nation’s leading plaintiffs' law firms and handles cases in all 50 states. We are very proud of our legal achievements, but equally self-respecting of our firm's reputation for providing personal attention to each and every client we represent.

