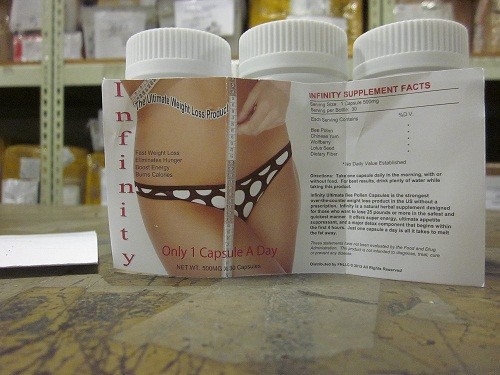
Infinity Bee Pollen is a diet pill that contains dangerous hidden ingredients. The FDA has detected sibutramine, a prescription appetite-suppressant that was banned in 2010. Infinity Bee Pollen can potentially cause heart attacks, stroke, high blood pressure, irregular heart rhythm, and other life-threatening side effects.
What You Can Do & How an Infinity Bee Pollen Lawsuit Can Help
The Schmidt Firm, PLLC is currently accepting Infinity Bee Pollen induced injury cases in all 50 states. If you or somebody you know was injured, you should contact our lawyers immediately for a free case consultation. Please use the form below to contact our Dietary Supplement Litigation Group or call us toll-free 24 hours a day at (866) 920-0753.
What is Infinity Bee Pollen?
Infinity Bee Pollen is a dietary supplement that is marketed as a “natural herbal supplement” that can burn fat, boost metabolism, suppress appetite, improve memory, and more.
FDA Safety Warning for Infinity Bee Pollen
In April 2014, the U.S. Food and Drug Administration (FDA) confirmed that Infinity Bee Pollen contains sibutramine, an illegal and hidden ingredient that is not listed on the label. They recommended against using the product.
According to the FDA Public Safety Alert:
“The product poses a threat to consumers because sibutramine is known to substantially increase blood pressure and/or pulse rate in some patients and may present a significant risk for patients with a history of coronary artery disease, congestive heart failure, arrhythmias, or stroke.”
Sibutramine can interact with other medications you are taking and cause life-threatening drug interactions.
What is Sibutramine?
Sibutramine is an appetite-suppressing drug that was originally approved in 1997. It was prescribed under the brand-name Meridia to treat obesity. The chemical structure is similar to amphetamines. Before it was withdrawn, sibutramine was sold by Abbott Laboratories.
Sibutramine Pulled off the Market
In October 2010, the FDA published a Safety Communication and pulled sibutramine off the market. They were concerned about the SCOUT clinical trial, which found that patients on sibutramine were 16% more likely to have a cardiovascular event than patients on a placebo.
Side Effects
- High blood pressure
- Irregular heart rhythm
- Increased heart rate
- Heart attack
- Stroke
- Cardiac arrest
- Death
Do I have an Infinity Bee Pollen Lawsuit?
The Schmidt Firm, PLLC is currently accepting Infinity Bee Pollen induced injury cases in all 50 states. If you or somebody you know was injured, you should contact our lawyers immediately for a free case consultation. Please use the form below to contact our Dietary Supplement Litigation Group or call us toll-free 24 hours a day at (866) 920-0753.
Attention Lawyers: We consider a referral from another law firm to be one of the greatest compliments. If your firm is interested in referring us a case or for us to send you a list of previous award judgments and/or average referral fees, please visit the Lawyer Referral section of our website.

 The Schmidt Firm, PLLC has been recognized as one of the nation’s leading plaintiffs' law firms and handles cases in all 50 states. We are very proud of our legal achievements, but equally self-respecting of our firm's reputation for providing personal attention to each and every client we represent.
The Schmidt Firm, PLLC has been recognized as one of the nation’s leading plaintiffs' law firms and handles cases in all 50 states. We are very proud of our legal achievements, but equally self-respecting of our firm's reputation for providing personal attention to each and every client we represent.

